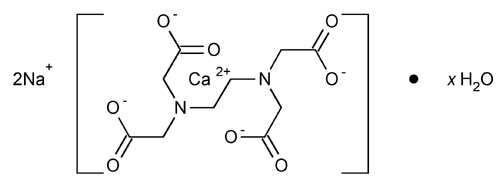
Edetate Calcium Disodium
Pharmacopeial Discussion Group Sign-Off Document
| Attributes | EP | JP | USP |
| Definition | + | + | + |
| Identification B | + | + | + |
| Identification C | + | + | + |
| pH | + | + | + |
| Purity (1) Chloride | + | + | + |
| Purity (2) Disodium edetate | + | + | + |
| Water | + | + | + |
| Assay | + | + | + |
Legend: + will adopt and implement;  will not stipulate.
will not stipulate.
Nonharmonized attributes: Clarity and/or color of solution, Heavy metals, Identification by IR absorption, Limit of nitrilotriacetic acid, Storage.
Specific local attributes: Description (JP), Iron (EP).
Calciate (2-), [[N,N¢-1,2-ethanediylbis[N-(carboxymethyl)glycinato]](4-)-N,N¢,O,O¢,ON,ON¢]-, disodium, hydrate, (OC-6-21)-.
Disodium[(ethylenedinitrilo)tetraacetato]calciate(2-) hydrate
Anhydrous
» Edetate Calcium Disodium contains not less than 98.0 percent and not more than 102.0 percent of C10H12CaN2Na2O8 (374.27), calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers. No storage requirements specified.
Identification—
B:
Dissolve 2 g in 10 mL of water, add 6 mL of lead (II) nitrate solution (33 in 1000), shake, and add 3 mL of potassium iodide TS: no yellow precipitate is formed. Make this solution alkaline by the addition of diluted ammonia solution (7 in 50), and add 3 mL of ammonium oxalate TS: a white precipitate is formed.
C:
Dissolve 0.5 g in 10 mL of water, and add 10 mL of potassium pyroantimonate TS: a white, crystalline precipitate is formed. The formation of the precipitate is accelerated by rubbing the inside wall of the test tube with a glass rod.
pH  791
791 :
between 6.5 and 8.0, in a solution (1 in 5).
:
between 6.5 and 8.0, in a solution (1 in 5).
Chloride  221
221 —
To 0.70 g add 20 mL of water and 30 mL of diluted nitric acid, allow to stand for 30 minutes, and filter. To 10 mL of the filtrate add water to make 50 mL, and perform the test using this solution as the test solution. Prepare the control solution with 0.40 mL of 0.01 M hydrochloric acid VS (not more than 0.10%).
—
To 0.70 g add 20 mL of water and 30 mL of diluted nitric acid, allow to stand for 30 minutes, and filter. To 10 mL of the filtrate add water to make 50 mL, and perform the test using this solution as the test solution. Prepare the control solution with 0.40 mL of 0.01 M hydrochloric acid VS (not more than 0.10%).
Water, Method I  921
921 :
between 5.0% and 13.0%, determined on 0.2 g.
:
between 5.0% and 13.0%, determined on 0.2 g.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Disodium edetate—
Dissolve 1.00 g of Edetate Calcium Disodium in 50 mL of water, add 5 mL of ammonia–ammonium chloride buffer TS, and 40 mg of eriochrome black T–sodium chloride indicator. Titrate with 0.01 M magnesium chloride VS until the color of the solution changes from blue to red-violet: not more than 3.0 mL of 0.01 M magnesium chloride VS is consumed (not more than 1.0%).
Limit of nitrilotriacetic acid—
Mobile phase, Cupric nitrate solution, Stock standard solution, and Chromatographic system—
Prepare as directed in the test for Limit of nitrilotriacetic acid under Edetate Disodium.
Resolution solution—
Using Edetate Calcium Disodium instead of Edetate Disodium, prepare as directed for Resolution solution in the test for Limit of nitrilotriacetic acid under Edetate Disodium.
Standard solution—
Transfer 1.0 g of Edetate Calcium Disodium to a 100-mL volumetric flask, add 100 µL of Stock standard solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to achieve complete solution.
Test solution—
Transfer 1.0 g of Edetate Calcium Disodium to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to achieve complete solution.
Procedure—
Proceed as directed for Procedure in the test for Limit of nitrilotriacetic acid under Edetate Disodium: the response of the nitrilotriacetic acid peak of the Test solution does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard solution and the Test solution (0.1%).
Assay—
Transfer about 500 mg of Edetate Calcium Disodium, accurately weighed, into a 200-mL volumetric flask. Dissolve in and dilute with water to volume, and mix. Transfer exactly 20 mL of this solution to 80 mL of water, and adjust with dilute nitric acid to a pH of 2 to 3. Add two drops of xylenol orange TS, and titrate with 0.01 M bismuth nitrate VS until the color of the solution changes from yellow to red. Each mL of 0.01 M bismuth nitrate VS is equivalent to 3.743 mg of C10H12CaN2Na2O8.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Kevin T. Moore, Ph.D.
Scientist 1-301-816-8369 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2228
Pharmacopeial Forum: Volume No. 32(4) Page 1335
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.