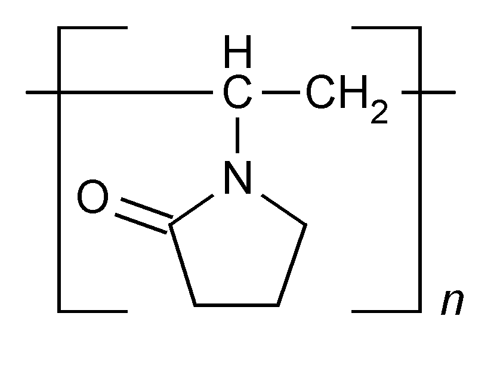
Crospovidone
» Crospovidone is a water-insoluble synthetic cross-linked homopolymer of N-vinyl-2-pyrrolidinone. It contains not less than 11.0 percent and not more than 12.8 percent of nitrogen (N), calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards  11
11 —
—
USP Crospovidone RS.
USP Crospovidone RS.
Identification—
B:
Suspend 1 g in 10 mL of water, add 0.1 mL of 0.1 N iodine, and shake for 30 seconds. Add 1 mL of starch TS, and shake: no blue color develops.
pH  791
791 :
between 5.0 and 8.0, in an aqueous suspension (1 in 100).
:
between 5.0 and 8.0, in an aqueous suspension (1 in 100).
Water, Method I  921
921 :
not more than 5.0%.
:
not more than 5.0%.
Residue on ignition  281
281 :
not more than 0.4%, a 2-g specimen being used.
:
not more than 0.4%, a 2-g specimen being used.
Water-soluble substances—
Transfer 25.0 g of Crospovidone to a 400-mL beaker, add 200 mL of water, and stir on a magnetic stirrer, using a 5-cm stirring bar, for 1 hour. Transfer to a 250-mL volumetric flask with the aid of about 25 mL of water, add water to volume, and mix. Allow the bulk of the solids to settle. Pass about 100 mL of the relatively clear supernatant through a membrane filter having a 0.45-µm porosity, protected against clogging by superimposing a membrane filter having a 3-µm porosity. While filtering, stir the solution above the filter manually or with a mechanical stirrer, taking care not to physically damage the membrane filter. Transfer 50.0 mL of the clear filtrate to a tared 100-mL beaker, evaporate to dryness, and dry at 110 for 3 hours: the weight of the residue does not exceed 75 mg (1.50%).
for 3 hours: the weight of the residue does not exceed 75 mg (1.50%).
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Vinylpyrrolidinone—
Suspend 4.0 g in 30 mL of water, stir for 15 minutes, centrifuge the suspension, and filter the slightly turbid upper layer through a sintered-glass, 10-µm filter. Stir the lower layer with 50 mL of water, centrifuge, and filter the upper layer through the same filter. Again stir the lower layer with 50 mL of water, and filter similarly. Add 0.5 g of sodium acetate to the combined filtrates, and titrate with 0.1 N iodine VS until the color of iodine no longer fades. Add 3.0 mL of 0.1 N iodine VS, allow to stand for 10 minutes, and titrate the excess iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ), using the same total volume of the same 0.1 N iodine VS, accurately measured, as was used for titrating the specimen. Before titrating the blank, adjust with acetic acid to the same pH as that of the specimen: not more than 0.72 mL of 0.1 N iodine is consumed, corresponding to not more than 0.1% of vinylpyrrolidinone.
), using the same total volume of the same 0.1 N iodine VS, accurately measured, as was used for titrating the specimen. Before titrating the blank, adjust with acetic acid to the same pH as that of the specimen: not more than 0.72 mL of 0.1 N iodine is consumed, corresponding to not more than 0.1% of vinylpyrrolidinone.
Nitrogen content—
Proceed as directed under Nitrogen Determination, Method II  461
461 , using about 0.1 g, accurately weighed, of Crospovidone. In the procedure, omit the use of hydrogen peroxide, and use 5 g of a powdered mixture of potassium sulfate, cupric sulfate, and titanium dioxide (33:1:1), instead of potassium sulfate and cupric sulfate (10:1). Heat until a clear, light green solution is obtained, and heat for an additional 45 minutes; and proceed as directed for Procedure, beginning with “Cautiously add to the digestion mixture 70 mL of water.”
, using about 0.1 g, accurately weighed, of Crospovidone. In the procedure, omit the use of hydrogen peroxide, and use 5 g of a powdered mixture of potassium sulfate, cupric sulfate, and titanium dioxide (33:1:1), instead of potassium sulfate and cupric sulfate (10:1). Heat until a clear, light green solution is obtained, and heat for an additional 45 minutes; and proceed as directed for Procedure, beginning with “Cautiously add to the digestion mixture 70 mL of water.”
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Kevin T. Moore, Ph.D.
Scientist 1-301-816-8369 |
(EM205) Excipient Monographs 2 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1219
Pharmacopeial Forum: Volume No. 28(4) Page 1257
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.