Wittig Reaction; Horner Reaction; Horner-Wadsworth-Emmons Reaction
G. Wittig, U. Schöllkopf, Ber. 87, 1318 (1954); G. Wittig, W. Haag, ibid. 88, 1654 (1955).
Alkene formation from carbonyl compounds and phosphonium ylides, proceeding primarily through the proposed betaine and/or oxaphosphetane intermediates. The stereoselectivity can be controlled by the choice of ylide, carbonyl compound, and reaction conditions:
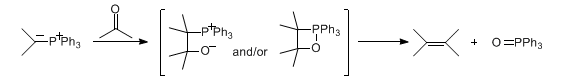
When the ylide is replaced with a phosphine oxide carbanion, the reaction is referred to as the Horner reaction: L. Horner et al., Ber. 91, 61 (1958); idem et al., ibid. 92, 2499 (1959).
When the ylide is replaced with a phosphonate carbanion, the reaction is referred to as the Horner-Emmons-Wadsworth reaction: W. S. Wadsworth, Jr., W. D. Emmons, J. Am. Chem. Soc. 83, 1733 (1961).
Application to the synthesis of β,γ-unsaturated amides: T. Janecki et al., Tetrahedron 51, 1721 (1995). Reviews: A. Maercker, Org. React. 14, 270-490 (1965); K. P. C. Vollhardt, Synthesis 1975, 765-780; W. S. Wadsworth, Jr., Org. React. 25, 73-253 (1977); I. Gosney, A. G. Rowley in Organophosphorus Reagents in Organic Synthesis, J. I. G. Cadogan, Ed. (Academic Press, New York, 1979) pp 17-153; B. E. Maryanoff, A. B. Reitz, Chem. Rev. 89, 863-927 (1989); S. E. Kelly, Comp. Org. Syn. 1, 755-782 (1991). Reviews of mechanistic studies: W. E. McEwen et al., ACS Symp. Ser. 486, 149-161 (1992); E. Vedejs, M. J. Peterson, Top. Stereochem. 21, 1-157 (1994). Cf. Peterson Reaction; Tebbe Reaction.