Pictet-Spengler Isoquinoline Synthesis
A. Pictet, T. Spengler, Ber. 44, 2030 (1911).
Formation of tetrahydroisoquinoline derivatives by condensation of β-arylethylamines with carbonyl compounds and cyclization of the Schiff bases formed:
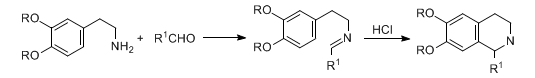
Reviews: W. M. Whaley, T. R. Govindachari, Org. React. 6, 151 (1951); R. A. Abramovitch, I. D. Spenser, Adv. Heterocycl. Chem. 3, 79 (1964); K. Stuart, R. Woo-Ming, Heterocycles 3, 223 (1975); D. Soerens et al., J. Org. Chem. 44, 535 (1979); H. Ernst et al., Ber. 114, 1894 (1981). Stereochemical study: E. Dominguez et al., Tetrahedron 43, 1943 (1987). Review of enantioselective modifications: M. D. Rozwadowski, Heterocycles 39, 903-931 (1994).