Lossen Rearrangement
W. Lossen, Ann. 161, 347 (1872); 175, 271, 313 (1874).
Conversion of a hydroxamic acid to an isocyanate via the intermediacy of its O-acyl, sulfonyl, or phosphoryl derivative. In the presence of amines, ureas are formed; in the presence of water, amines containing one less carbon than the starting material, are generated:
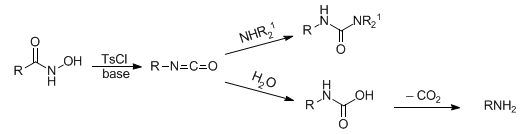
Reviews: H. L. Yale, Chem. Rev. 33, 209 (1943); L. Bauer, O. Exner, Angew. Chem. Int. Ed. 13, 376 (1974); T. Shiori, Comp. Org. Syn. 6, 821-825 (1991). Reaction conditions leading to the formation of ureas: J. Pihuleac, L. Bauer, Synthesis 1989, 61; extention to N-phosphinoylhydroxylamines: J. Fawcett et al., Chem. Commun. 1992, 227; C. J. Salomon, E. Breuer, J. Org. Chem. 62, 3858 (1997); to sulfonyloxy imides: D. A. Casteel et al., Heterocycles 36, 485 (1993). Modifications: J. A. Stafford et al., J. Org. Chem. 63, 10040 (1998); R. Anilkumar et al., Tetrahedron Lett. 41, 5291 (2000). Cf. Curtius Rearrangement; Hofmann Reaction; Schmidt Reaction.