Benzidine Rearrangement; Semidine Rearrangement
A. W. Hofmann, Proc. Roy. Soc. London 12, 576 (1863); P. Jacobson et al., Ber. 26, 688 (1893).
Acid-catalyzed rearrangement of hydrazobenzenes to 4,4′-diaminobiphenyls. If the hydrazobenzene contains a para substituent, then the favored product is p-aminodiphenylamine (Semidine rearrangement):
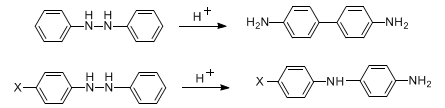
D. L. H. Williams, Compr. Chem. Kinet. vol. 13, C. H. Bamford, C. F. H. Tipper, Eds. (Elsevier, New York, 1972) pp 437-448; R. A. Cox, E. Buncel, The Chemistry of Hydrazo, Azo and Azoxy Groups pt. 2, S. Patai, Ed. (Wiley, New York, 1975) pp 775-807. Mechanistic studies: H. J. Shine et al., J. Am. Chem. Soc. 103, 955 (1981); 104, 5184 (1982); 106, 7077 (1984). Synthetic applications: T. Nozoe et al., Chem. Lett. 1986, 1577; K. H. Park, J. S. Kang, J. Org. Chem. 62, 3794 (1997).