Hydroboration Reaction
H. C. Brown, B. C. Subba Rao, J. Am. Chem. Soc. 78, 5694 (1956); J. Org. Chem. 22, 1135, 1136 (1957).
Addition of boron hydrides to alkenes, allenes, and alkynes to form organoboranes, such that boron adds to the less substituted carbon. Attack usually takes place on the less hindered side in a cis fashion:
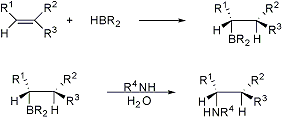
Diastereofacial and regioselectivity study: B. W. Gung et al., Synth. Commun. 24, 167 (1994). Methods development for asymmetric synthesis: U. P. Dhokte, H. C. Brown, Tetrahedron Letters 35, 4715 (1994). Application to hydration: G. Zweifel, H. C. Brown, Org. React. 13, 1-54 (1963). General reviews: H. O. House, Modern Synthetic Reactions (W. A. Benjamin, Menlo Park, California, 2nd ed., 1972) pp 106-130; K. Smith, A. Pelter, Comp. Org. Syn. 8, 703-731 (1991). Reviews of asymmetric synthesis: H. C. Brown, Tetrahedron 37, 3547-3587 (1981); K. Burgess, M. J. Ohlmeyer, Adv. Chem. Ser. 230, 163-177 (1992). Cf. Suzuki Coupling.