Combes Quinoline Synthesis
A. Combes, Bull. Soc. Chim. France 49, 89 (1888).
Formation of quinolines by condensation of β-diketones with primary arylamines followed by acid-catalyzed ring closure of the intermediate Schiff base:
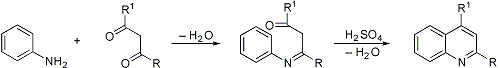
W. S. Johnson, F. J. Matthews, J. Am. Chem. Soc. 66, 210 (1944); F. W. Bergstrom, Chem. Rev. 35, 156 (1944); J. C. Perche et al., J. Chem. Soc. Perkin Trans. I 1972, 260; J. Born, J. Org. Chem. 37, 3952 (1972). Cf. Conrad-Limpach Reaction; Doebner Reaction.