Wagner-Meerwein Rearrangement
G. Wagner, J. Russ. Phys. Chem. Soc. 31, 690 (1899); H. Meerwein, Ann. 405, 129 (1914).
Carbon-to-carbon migration of alkyl, aryl or hydride ions. The original example is the acid-catalyzed rearrangement of camphene hydrochloride to isobornyl chloride:
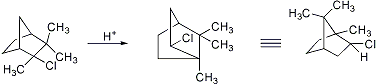
C. Le Drian, P. Vogel, Helv. Chim. Acta 70, 1703 (1987); M. Asaoka, H. Takei, Tetrahedron Letters 28, 6343 (1987); L. U. Román et al., J. Org. Chem. 56, 1938 (1991). Review of applications to alcohols: Y. Pocker in Molecular Rearrangements Part 1, P. de Mayo, Ed. (Wiley-Interscience, New York, 1963) pp 6-15; to bicyclic systems: J. Berson, ibid. 111-231; to terpenes: J. F. King, P. de Mayo, ibid. 813-840; to alkaloids: E. W. Warnhof, ibid. 842-879; to steroids: N. L. Wendler, ibid. 1020-1028. Reviews: R. L. Cargill et al., Accts. Chem. Res. 7, 106-113 (1974); H. Hogeveen, E. M. G. A. Van Kruchten, Top. Curr. Chem. 80, 89-124 (1979); J. R. Hanson, Comp. Org. Syn. 3, 705-719 (1991). Cf. Demjanov Rearrangement; Nametkin Rearrangement; Retropinacol Rearrangement.