Pummerer Rearrangement
R. Pummerer, Ber. 43, 1401 (1910).
Rearrangement of sulfoxides to α-acyloxythioethers in the presence of acyclic anhydrides. When nucleophiles other than those derived from the anhydride are present, different functionalization is achieved:
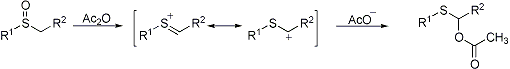
Diastereoselectivity in the preparation of 4-phenyl-4-butanolides: H. Su et al., Bull. Chem. Soc. Japan 66, 2603 (1993). Application to vinyl sulfoxides (the additive Pummerer reaction): D. Craig, K. Daniels, Tetrahedron 49, 11263 (1993). Regiospecific cyclization: G. Majumdar, D. Mal, Indian J. Chem. 33B, 700 (1994). Asymmetric synthesis: Y. Kita et al., Tetrahedron Letters 35, 3575 (1994). Reviews: O. DeLucchi et al., Org. React. 40, 157-405 (1991); D. S. Grierson, H.-P. Husson, Comp. Org. Syn. 6, 924-937 (1991).