Jacobsen Epoxidation
W. Zhang et al., J. Am. Chem. Soc. 112, 2801 (1990); E. N. Jacobsen et al. ibid. 113, 7063 (1991).
Chiral (salen)manganese(III)-catalyzed asymmetric epoxidation of alkenes. Enantio- and diastereo- selectivity depend strongly on the nature of the substrate:
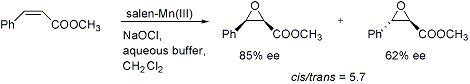
Methods development: E. N. Jacobsen et al., Tetrahedron 50, 4323 (1994); S. Chang et al., J. Am. Chem. Soc. 116, 6937 (1994); B. D. Brandes, E. N. Jacobsen, J. Org. Chem. 59, 4378 (1994). Large-scale preparation of ligand: J. F. Larrow et al., ibid. 1939. Review: E. N. Jacobsen, “Asymmetric Catalytic Epoxidation of Unfunctionalized Olefins” in Catalytic Asymmetric Synthesis, I. Ojima, Ed. (VCH, New York, 1993) pp 159-202. For parallel studies, see N. Hosoya et al., Synlett 1993, 641; H. Sasaki et al., ibid. 1994, 356. Mechanistic study: D. L. Hughes et al., J. Org. Chem. 62, 2222 (1997). Application: P. S. Savle et al., Tetrahedron Asymmetry 9, 1843 (1998). Review: T. Flessner et al., J. Prakt. Chem. 341, 436-444 (1999).