Diels-Alder Reaction
O. Diels, K. Alder, Ann. 460, 98 (1928); 470, 62 (1929); Ber. 62, 2081, 2087 (1929).
The 1,4-addition of the double bond of a dienophile to a conjugated diene to generate a six-membered ring, such that up to four new stereocenters may be created simultaneously. The [4+2]-cycloaddition usually occurs with high regio- and stereoselectivity:
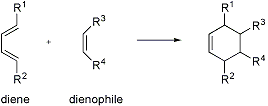
Heteroatomic analogs of the diene (e.g., CHR=CR-CR=O, O=CR-CR=O, and RN=CR-CR=NR) and dienophile (e.g., RN=NR, R2C=NR, and RN=O) may also serve as reactants.
Early reviews: M. C. Kloetzel, Org. React. 4, 1-59 (1948); H. L. Holmes ibid. 60-173; L. W. Butz, A. W. Rytina, ibid. 5, 136-192 (1949). Intermolecular reactions: W. Oppolzer, Comp. Org. Syn. 5, 315-399 (1991). Intramolecular reactions: E. Ciganek, Org. React. 32, 1-374 (1984); W. R. Rousch, Comp. Org. Syn. 5, 513-550 (1991). Use of heterodienophiles: S. M. Weinreb, ibid. 401-449. Use of nitroso dienophiles: J. Streith, A. DeFoin, Synthesis 1994, 1107-1117. Use of heterodienes: D. L. Boger, ibid, 451-512. Review of diastereoselectivity: J. M. Coxon et al., “Diastereofacial Selectivity in the Diels-Alder Reaction” in Advances in Detailed Reaction Mechanisms 3, 131-166 (1994); T. Oh, M. Reilly, Org. Prep. Proceed. Int. 26, 131-158 (1994); H. Waldmann, Synthesis 1994, 535-551. Cf. Wagner-Jauregg Reaction.